PATIENT SERIES: PATIENTS WITH THERAPEUTIC COMPROMISE – SAFETY ISSUES
In this section
Characteristics
Some schizophrenia patients with therapeutic compromise experience adverse effects of antipsychotic (AP) medication.1 These patients may require a switch in medication.1 Patients in this population are characterised by greater instability of their condition and a high risk of relapse, resulting from poor treatment compliance due primarily to adverse effects, even in patients who are otherwise treatment-responsive.1
Intolerable adverse effects of APs, caused either by a single AP or the combination of different medications, can contribute to a deterioration in the patient’s medical condition.2 Severe AP side effects include hyperprolactinaemia and subsequently, sexual dysfunction in both men and women.3 Cardiac issues, such as QT prolongation, are frequent side effects of antipsychotic medication, and are associated with a risk of death: sudden cardiac death was found to be twice as prevalent in patients taking APs as in the general population.4 Although observed previously with first-generation antipsychotics, metabolic side effects, such as hyperlipidaemia (blood cholesterol and triglyceride elevation), hyperglycaemia, diabetes mellitus and weight gain, became more prominent with second-generation antipsychotics.5 These side effects lead to potential non-adherence with medication, and therefore constitute a therapeutic compromise in patients.5 Sedation is a frequently reported side effect of AP medication that has been associated with poor treatment-adherence.4 Akathisia is a another side effect that often occurs when patients are treated with first-generation APs.5
Clinical management
Treatment goals for patients with therapeutic compromise experiencing safety problems include minimising side effects, such as hyperprolactinaemia, sexual dysfunction3, serious cardiac adverse events4, metabolic changes5, sedation4, EPS and akathisia.5 In such cases, a switch in AP medication is usually warranted.1
Although AP medications are a recognised contributing factor, physical inactivity and poor dietary habits also play a key role in the development of obesity, diabetes mellitus and metabolic syndrome.2 Therefore, lifestyle interventions, including the establishment of good nutritional and physical exercise habits, could help minimise these serious health conditions.2 Studies have shown that lifestyle interventions are efficacious in treating obesity, which consequently helps reduce the chances of developing diabetes mellitus, hyperglycaemia and hyperlipidaemia.2 Furthermore, as metabolic diseases contribute to cardiovascular diseases, lifestyle interventions also have the potential to reduce the risk of developing these latter conditions.2
Cariprazine’s place in the treatment of patients with therapeutic compromise experiencing safety problems
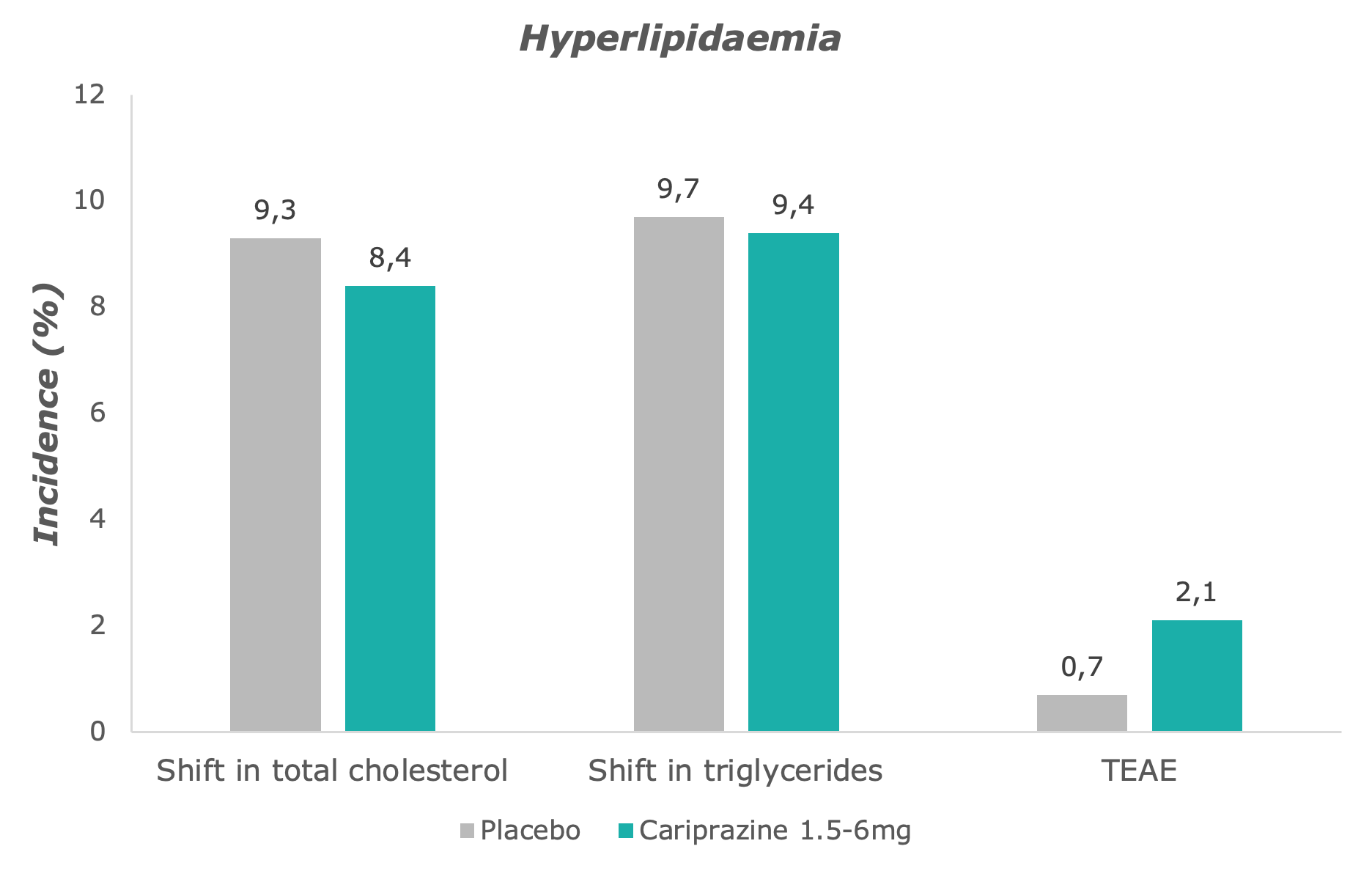
On the whole, cariprazine is generally safe and well tolerated by patients.6 Slightly more cariprazine-treated patients had a shift in total cholesterol, while shifts in triglycerides in the cariprazine group was comparable to the placebo group.7 The incidence of hyperlipidaemia-related AEs was only slightly higher in all cariprazine-treated patients than in placebo-treated patients.7
Adapted from: Barabássy, Á. et al. Safety and Tolerability of Cariprazine in Patients with Schizophrenia: A Pooled Analysis of Eight Phase II/III Studies. Neuropsychiatr. Dis. Treat. 17, 957–970 (2021)
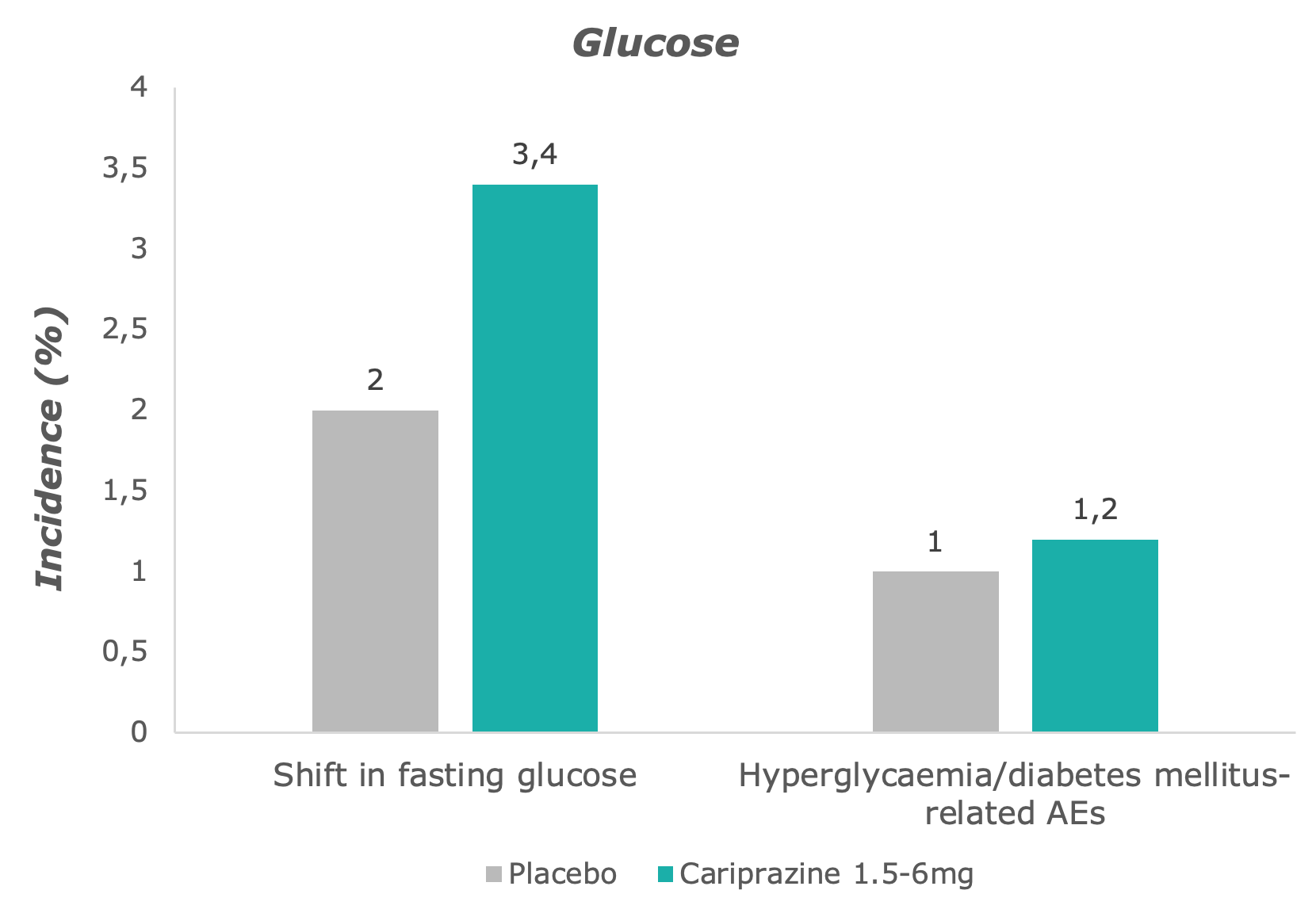
Compared to placebo, slightly more cariprazine-treated patients shifted from normal to high levels of fasting glucose – however, levels remained stable over time in the cariprazine group.7
Adapted from: Barabássy, Á. et al. Safety and Tolerability of Cariprazine in Patients with Schizophrenia: A Pooled Analysis of Eight Phase II/III Studies. Neuropsychiatr. Dis. Treat. 17, 957–970 (2021)
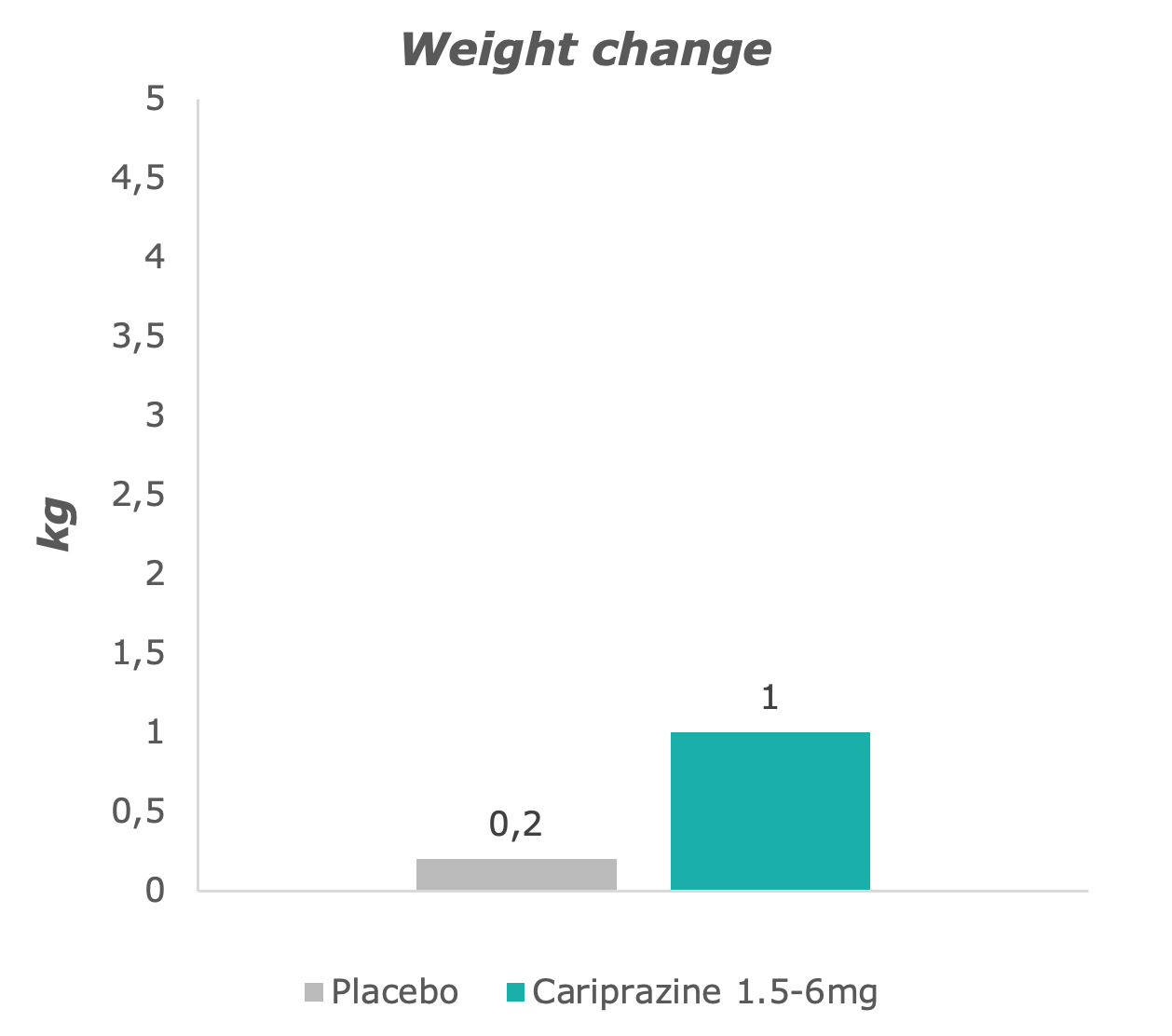
Slightly greater weight change was observed in the cariprazine-treated group than in placebo-treated patients.7
Adapted from: Barabássy, Á. et al. Safety and Tolerability of Cariprazine in Patients with Schizophrenia: A Pooled Analysis of Eight Phase II/III Studies. Neuropsychiatr. Dis. Treat. 17, 957–970 (2021)
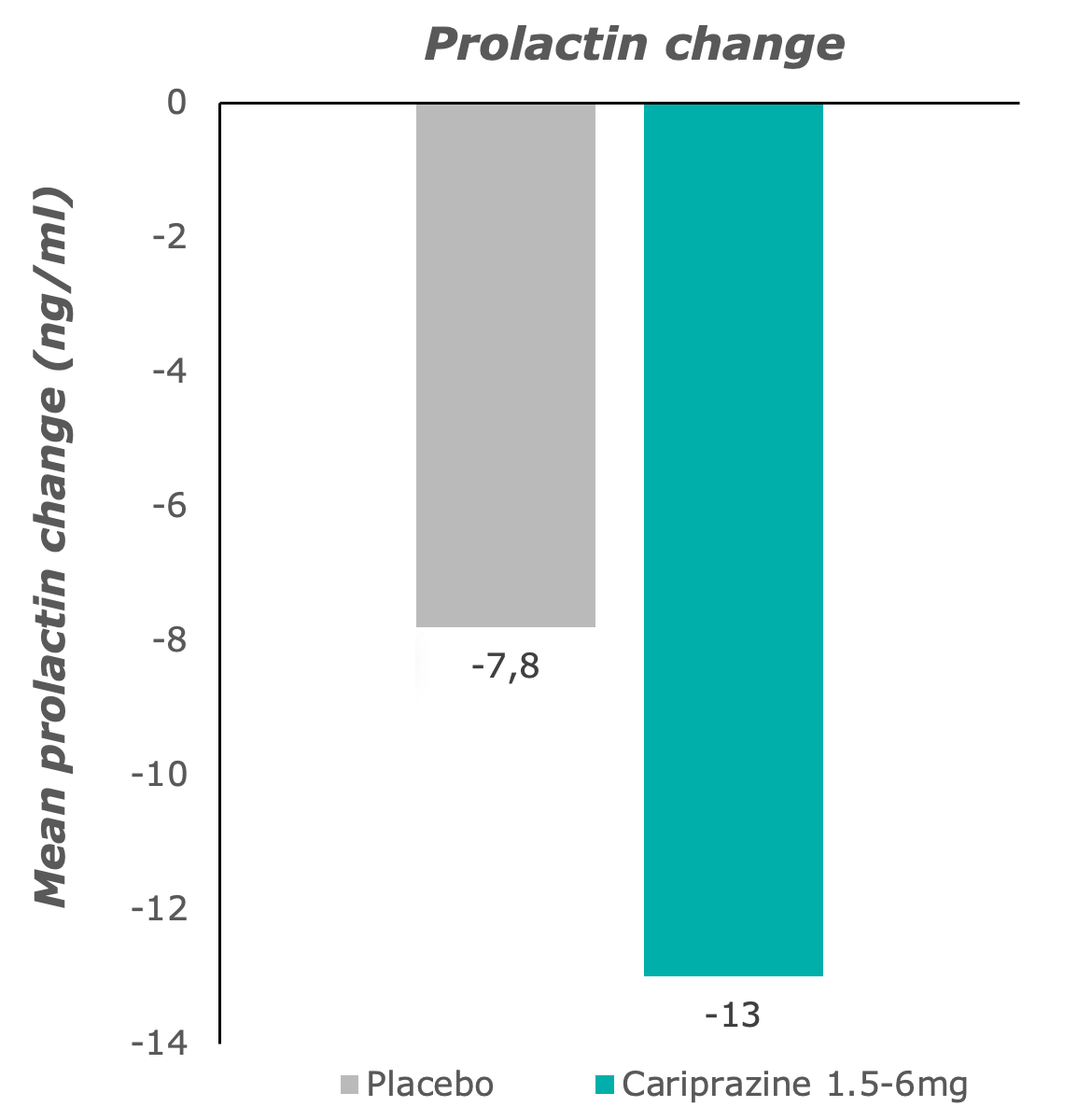
Hyperprolactinaemia was not observed with cariprazine treatment: prolactin decreased to normal ranges.7
Adapted from: Barabássy, Á. et al. Safety and Tolerability of Cariprazine in Patients with Schizophrenia: A Pooled Analysis of Eight Phase II/III Studies. Neuropsychiatr. Dis. Treat. 17, 957–970 (2021)
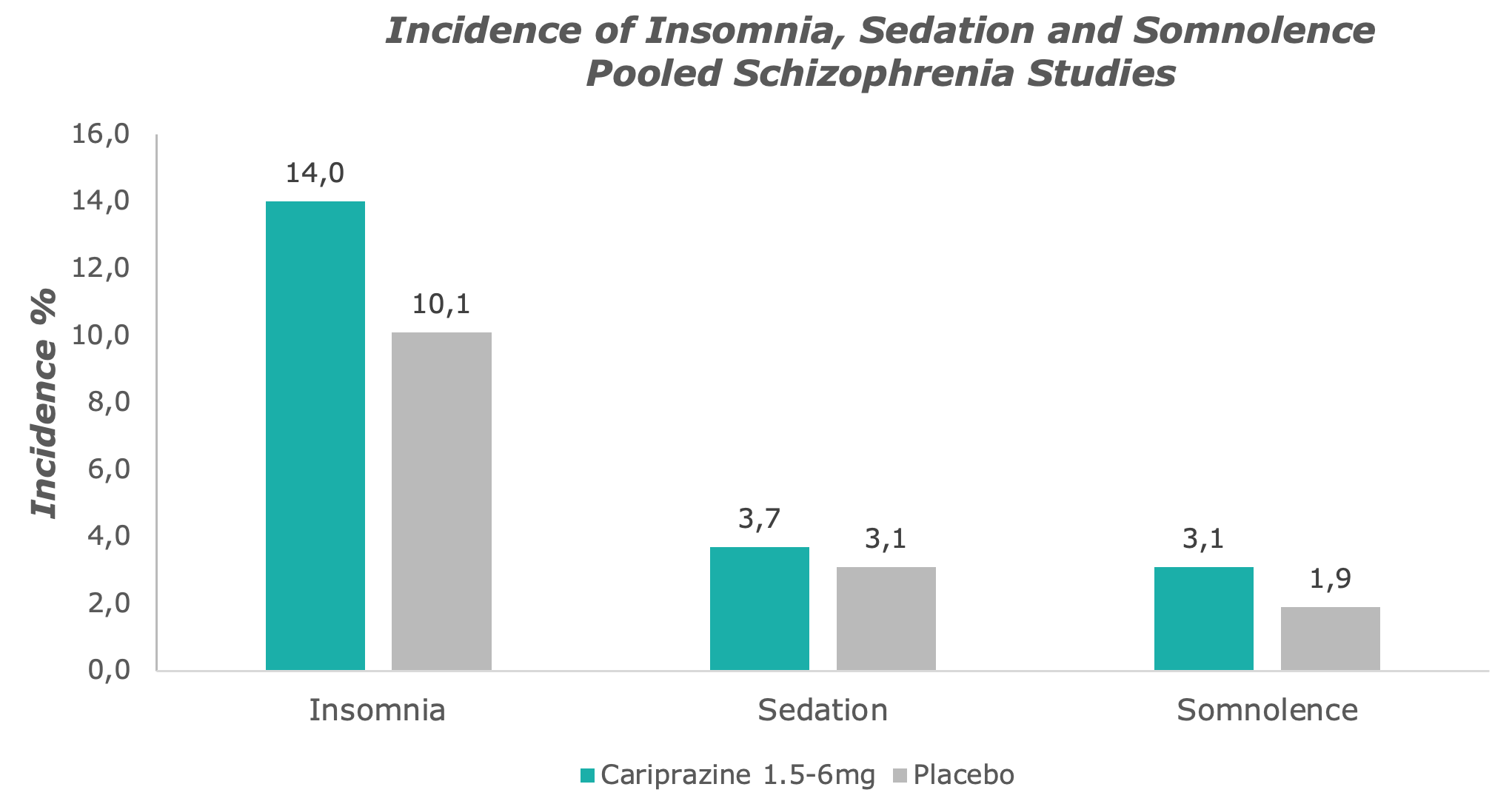
Cariprazine causes little sedation and drowsiness, rather it is an activating substance.7
Adapted from: Barabássy, Á. et al. Safety and Tolerability of Cariprazine in Patients with Schizophrenia: A Pooled Analysis of Eight Phase II/III Studies. Neuropsychiatr. Dis. Treat. 17, 957–970 (2021)
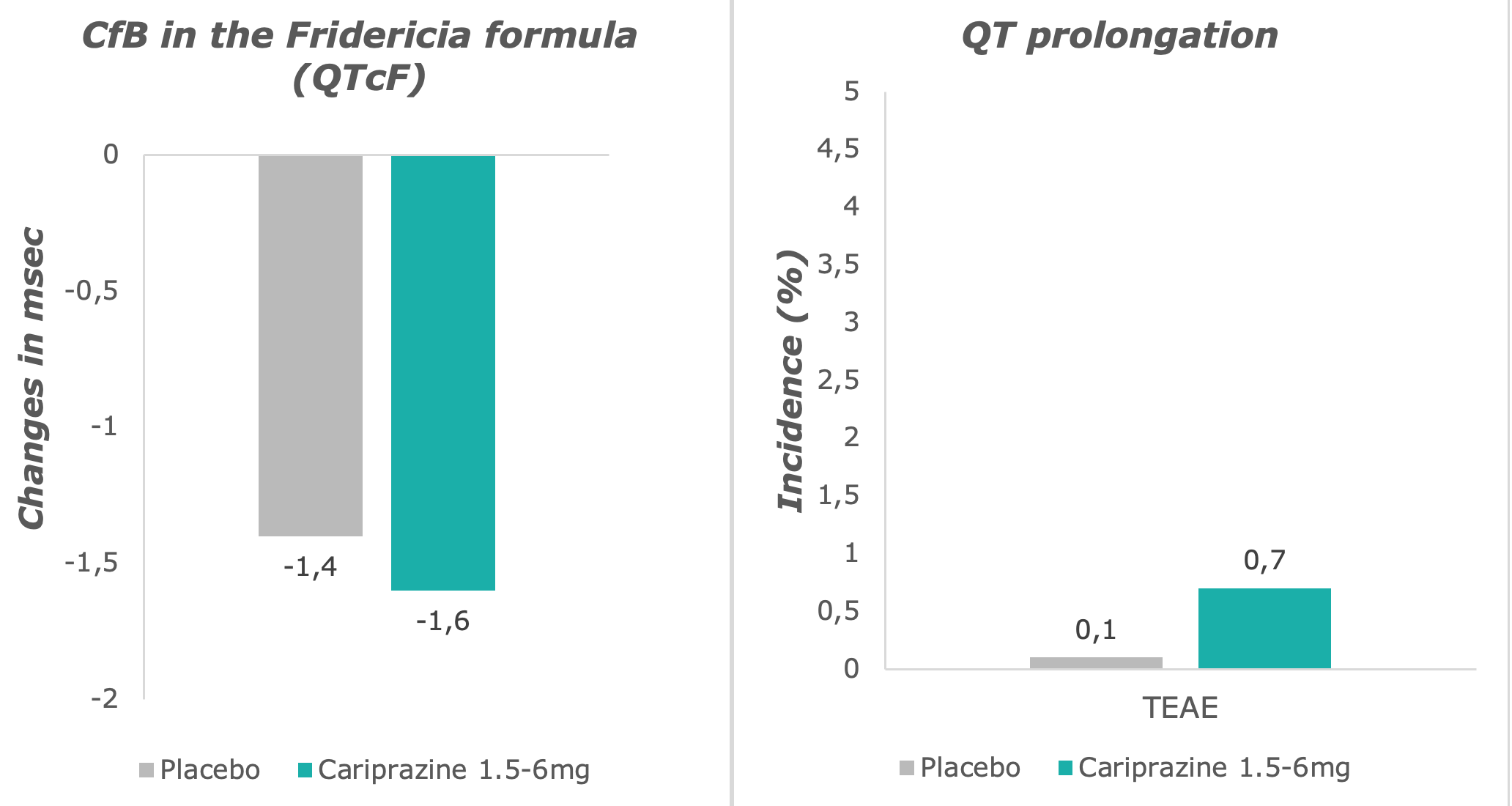
Potentially clinically significant QT prolongation and reports of adverse events were very low in the cariprazine group, and comparable with the placebo group.7
Adapted from: Barabássy, Á. et al. Safety and Tolerability of Cariprazine in Patients with Schizophrenia: A Pooled Analysis of Eight Phase II/III Studies. Neuropsychiatr. Dis. Treat. 17, 957–970 (2021)
Switching
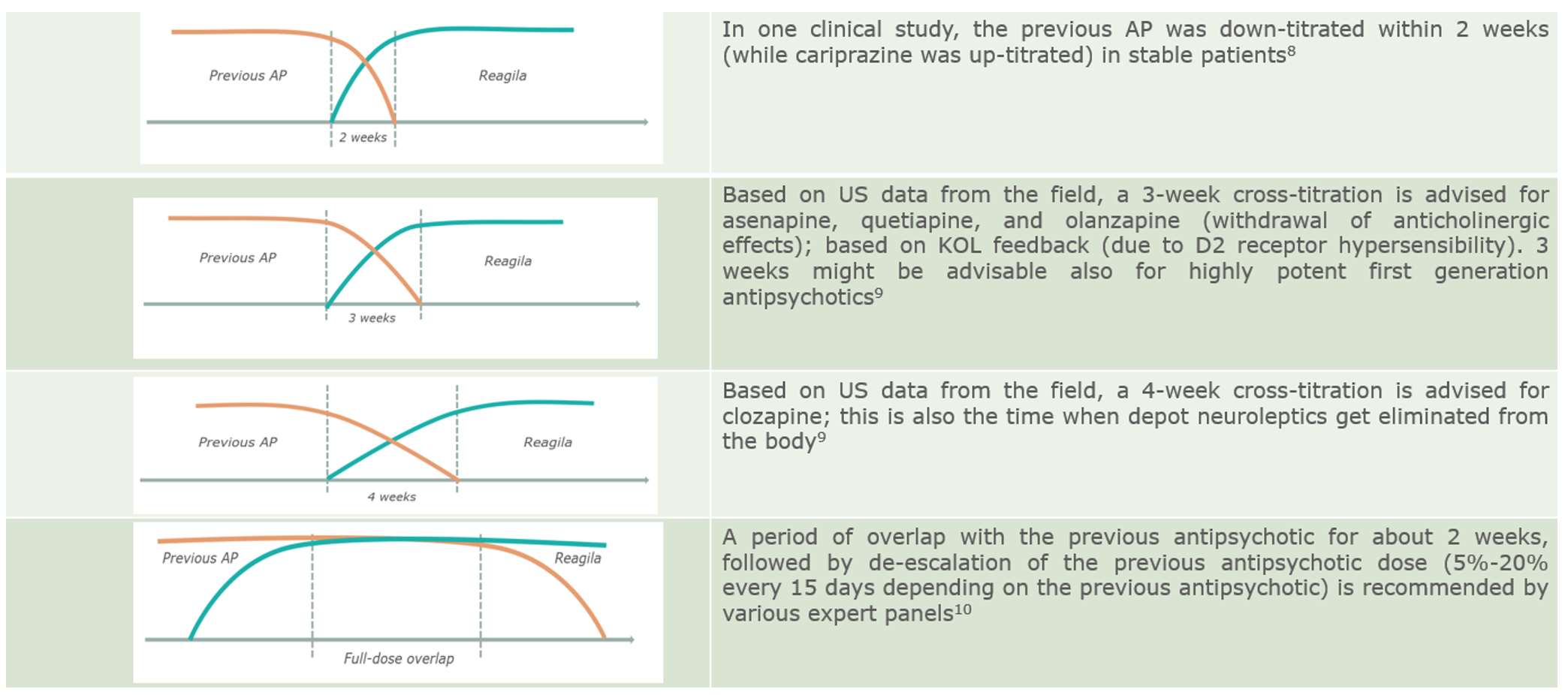
In patients with therapeutic compromise, slower switching strategies and gradual cross-titration may be indicated.1 Weekly dose-increases of cariprazine in 1.5 mg increments represent a slower up-titration of cariprazine in stable patients with schizophrenia.8 On the other hand, dose decreases in the previous antipsychotics at any time (1 week9, 2 weeks8, 3 weeks9) up to 4 weeks9 – or even a full dose overlap for a certain time – are recommended when switching stable patients.10
COD. 300021/R59. Submitted to AIFA on xx/xx/xxxx
References
- Bobo, W. Switching Antipsychotics: Why, When, and How? Psychiatr. Times 30, (2013).
- Chacón, F., Mora, F., Gervás-Ríos, A. & Gilaberte, I. Efficacy of lifestyle interventions in physical health management of patients with severe mental illness. Ann. Gen. Psychiatry 10, 22 (2011).
- Park, Y. W., Kim, Y. & Lee, J. H. Antipsychotic-Induced Sexual Dysfunction and Its Management. World J. Mens. Health 30, 153 (2012).
- Muench, J. & Hamer, A. M. Adverse effects of antipsychotic medications. Am. Fam. Physician 81, 617–622 (2010).
- Hansen, T. E., Snyder, K., Messamore, E. & Hoffman, W. F. Metabolic side effects of antipsychotic medications: Clinical laboratory implications. Lab. Med. 35, 625–627 (2004).
- Nasrallah, H. A. et al. The safety and tolerability of cariprazine in long-term treatment of schizophrenia: A post hoc pooled analysis. BMC Psychiatry 17, 1–13 (2017).
- Barabássy, Á. et al. Safety and Tolerability of Cariprazine in Patients with Schizophrenia: A Pooled Analysis of Eight Phase II/III Studies. Neuropsychiatr. Dis. Treat. 17, 957–970 (2021).
- Németh, G. et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389, 1103–1113 (2017).
- Stahl, S. M. Prescriber’s Guide: Antipsychotics (Stahl’s Essential Psychopharmacology). (Cambridge University Press, 2017).
- Fagiolini, A., Brugnoli, R., Di Sciascio, G., De Filippis, S. & Maina, G. Switching antipsychotic medication to aripiprazole: Position paper by a panel of Italian psychiatrists. Expert Opin. Pharmacother. 16, 727–737 (2015).
[dc-hide-content]PATIENT WITH EARLY PSYCHOSIS[/dc-hide-content]PATIENT WITH EARLY PSYCHOSIS
[dc-hide-content](COD. 300020/R41. Submitted to AIFA on 12 October 2020). This article by D. De Berardis et al. summarizes a case report of early schizophrenia treated successfu[/dc-hide-content]This article by D. De Berardis et al. summarizes a case report of early schizophrenia treated successfully with our product monotherapy.
more…[dc-hide-content]CARIPRAZINE ADD-ON: ANOTHER CASE[/dc-hide-content]CARIPRAZINE ADD-ON: ANOTHER CASE
[dc-hide-content](COD. 300020/R43. Submitted to AIFA on 12 October 2020). This article by De Berardis et al. presents a case of schizophrenia successfully treated with cariprazi[/dc-hide-content]This article by De Berardis et al. presents a case of schizophrenia successfully treated with our product as an add-on therapy in inadequate clozapine response.
more…